
Chapter 4
Playing With Fire » Fire Without Flame

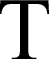 ake a torch to your catalytic converter to
understand what burning really means. To a chemist, burning means the rapid combination of a fuel with oxygen,
called oxidation. You might say, for instance, "Oh, no, we didn't have a fire
at the nuclear power plant, we just had a 'rapid oxidation event,' " a phrase
that won officials at Three Mile Island the Doublespeak Award in 1979.
ake a torch to your catalytic converter to
understand what burning really means. To a chemist, burning means the rapid combination of a fuel with oxygen,
called oxidation. You might say, for instance, "Oh, no, we didn't have a fire
at the nuclear power plant, we just had a 'rapid oxidation event,' " a phrase
that won officials at Three Mile Island the Doublespeak Award in 1979.
When gasoline or propane burns in air, the result is a bright flame-fire in the traditional sense. But there are other ways to combine these fuels rapidly with air. The catalytic converter in your car is a perfect example. Most contain a ceramic honeycomb studded with microscopic particles of platinum, palladium or rhodium. Among other functions, these metals split oxygen molecules that land on their surface into more-reactive single oxygen atoms, which can combine with fuel at a much lower temperature than in a flame.







 Rusting Aluminum
Rusting Aluminum








